|
|
From Yana (reposted from e-mail sent to LWAB members):A friend of mine sent it to me. She said that I can share this
information. Most people are worried about some ingedients that are in
the vaccine. Mostly Thiomersal and Squalene. Here are her findings.
See below for some of the research
I pulled off of reputable websites and an attachment which goes into
the clinical background of the H1N1 vaccine including the results of
studies conducted and the ingredients.
Thimerosal is an
ingrediant used in the regular flu vaccine as well, and based on my own
research i believe it's harsh to say that it causes "brain damage".
1. Thiomersal
is an organic mercurial compound that has been used for over 60 years
as an antimicrobial agent in vaccines and other pharmaceutical products
to prevent unwanted bacterial and fungal growth in the opened vaccine
vial. It is also present in the childhood vaccines we give our children
(DTP, MMR, HiB etc...)
2. Squalene is a natural organic compound originally obtained for commercial purposes primarily from shark liver oil, though botanic sources (primarily vegetable oils) are used as well, including amaranth seed, rice bran, wheat germ, and olives. All higher organisms produce squalene, including humans. It is a hydrocarbon and a triterpene. Squalene is a natural and vital part of the synthesis of cholesterol, steroid hormones, and vitamin D in the human body.[1] Squalene is used in cosmetics, and more recently as an immunologic adjuvant in vaccines.
Thimerosal in Canadian Vaccines
Thimerosal is an organic mercury compound that is
an effective preservative. It is used in some vaccines and in
pharmaceutical and other consumer products, such as cosmetics. First
introduced in the 1930s, it prevents bacterial and fungal contamination
of these products. It may be used during the manufacture of vaccine to
inactivate organisms or added as a preservative to prevent
contamination of the product after manufacture, particularly in
multi-dose vials.
Thimerosal is metabolized to ethylmercury (CH3CH2Hg+2)
and thiosalicylate. It contains 49.6% mercury by weight. In the final
preparation of vaccines, the concentration of thimerosal is small,
measured in micrograms (µg), one millionth of a gram. When a person is
immunized with a vaccine that contains thimerosal, the resultant
concentration of metabolized ethylmercury is reduced even further as it
is diluted in the body.
The detrimental health effects of high-dose
exposure to mercury have been well studied. In addition, acute
accidental poisoning episodes with very high doses of thimerosal and
improperly prepared medicines containing thimerosal have been
documented. However, the amount of thimerosal in vaccines is small, and
no studies have documented any associated adverse effects beyond the
hypersensitivity reactions noted in the next section.
Immediate hypersensitivity, including anaphylaxis
and immune-complex-mediated disorders, has been reported with some
products that contain thimerosal, but it is uncertain whether
thimerosal was the responsible agent. Anaphylaxis has not been proven
to occur as a result of thimerosal in vaccines and thus remains a
theoretical risk.
If an individual is suspected of being
hypersensitive to thimerosal, the nature of the hypersensitivity
reaction must be characterized before a vaccine containing thimerosal
is administered. If there is no proven history of hypersensitivity,
vaccination can proceed without particular precaution. If there is a
definite history of anaphylaxis to thimerosal, vaccines containing this
component should not be given. Although such reactions have never been
reported, prior history of erythema multiforme, Stevens-Johnson
syndrome or toxic epidermal necrolysis from thimerosal exposure would
be an absolute contraindication to future exposure. In cases of proven
delayed hypersensitivity to thimerosal, vaccination can proceed but the
patient should be advised that long-lasting local or systemic cutaneous
reactions can occur.
Thimerosal Content of Vaccines Used in Canada
Vaccines approved for use in Canada may contain the following:
- no thimerosal: these are single-dose preparations in which
thimerosal has not been used in any part of the manufacturing process;
- trace amounts of thimerosal (< 1.0 µg/dose) if the
preservative has been used in the production process but not added to
serve a preservative function in the final product;
- thimerosal added as a preservative. Such vaccines are
typically those supplied in multi-dose vials with thimerosal added in
varying concentrations to prevent contamination with other serious
infectious agents. The amount of mercury per dose varies from 2 to 50
µg per 0.5 mL dose.
Table 1
identifies vaccines currently approved for use in Canada in terms of
thimerosal content. Most of the listed vaccines containing thimerosal
as a preservative are not widely used, either because they have been
replaced by newer vaccines or have very specialized use (e.g. DT
adsorbed). Some, such as influenza vaccines, are used in
provincial/territorial immunization programs. A more detailed table of
vaccine contents is published in the 2006 Canadian Immunization Guide(8), and updates will be posted, as appropriate, at <www.naci.gc.ca>. These would include any changes to the preservative content of vaccines listed in Table 1 as well as changes in vaccines approved for use in Canada after this statement has been published.
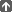
Table 1. Thimerosal content of vaccines marketed in Canada as of May 1, 2007*
|
Preservative amounts
(2 to 50 ug/dose)
|
Trace amounts
(< 1 ug/dose of vaccine)
|
None
|
- Epaxal
- Fluviral
- JE-VAX
- Menomune A/C/Y/W-135 (multidose vial)
- Recombivax HB (multidose vial)
- Tetanus toxoid (adsorbed)
- Vaxigrip (multidose vial)
|
- Engerix B (multidose)
- Infanrix-hexa
- Twinrix
- Twinrix Junior
|
- Actacel
- Adacel
- Avaxim
- Avaxim = pediatric
- BCG
- Boostrix
- DT Polio Absorbed
- Dukoral
- Engerix B single dose vial
- Eolarix
- FSME-IMMUN
- Gardasil
- Havrix
- Hiberix
- Imovax Polio
- Imovax Rabies
- Inactivated Poliomyelitis Vaccine (IPV)
- Infanrix
- Infanrix-Hib
- Infanrix-IPV
- Infanrix-IPV/Hib
- Influvac
- Liquid Pedvax
- Menactra
- Meningitec
- Menjugate
- Menomune A/C
- Menomune A/C/Y/W-135 single dose vial
- MMR II
- Mutacol
- Neisvac-C
- Pediacel
- Pediarix
- Pentacel
- Pneumo 23
- Pneumovax 23
- Prevnar
- Priorix
- Quadracel
- RabAvert
- Recombivax HB single dose vial
- RotaTeq
- Td Adsorbed
- Td Polio Adsorbed
- Tripacel
- Typherix
- Typhim Vi
- Vaqta
- Varilrix
- Varivax III
- Vaxigrip singledose vial
- ViVaxim
- Vivotif
- Vivotif L
- YF-VAX
|
*A more detailed and, as appropriate, updated table of vaccine
contents, including preservatives such as thimerosal, can be found at
<www.naci.gc.ca>.
|
Squalene-based adjuvants in vaccines
What is squalene?
- Squalene is a naturally occurring substance found in plants,
animals, and humans. It is manufactured in the liver of every human
body and circulates in our bloodstream.
- Squalene is also found in a variety of foods, cosmetics, over-the-counter medications, and health supplements.
- Squalene is commercially extracted from fish oil, and in
particular shark liver oil. Squalene used in pharmaceutical products
and vaccines is purified from this source.
Is there squalene in vaccines?
- Since 1997, an influenza vaccine (FLUAD, Chiron) which contains
about 10 mg of squalene per dose, has been approved in health agencies
in several European countries. Squalene is present in the form of an
emulsion and is added to make the vaccine more immunogenic.
- Squalene is being added to improve the efficacy of several
experimental vaccines including pandemic flu and malaria vaccines which
are being developed.
Why is squalene added to vaccines?
- Squalene is a component of some adjuvants that are added to vaccines to enhance the immune response.
- MF59, an adjuvant produced by Novartis and added to the FLUAD flu vaccine, is such an example.
- Squalene by itself is not an adjuvant, but emulsions of squalene with surfactants do enhance the immune response.
What is known about the safety of squalene in vaccines?
- Twenty two million doses of Chiron's influenza vaccine (FLUAD) have
been administered safely since 1997. This vaccine contains about 10mg
of squalene per dose. No severe adverse events have been associated
with the vaccine. Some mild local reactogenicity has been observed.
- Clinical studies on squalene-containing vaccines have been done in infants and neonates without evidence of safety concerns.
Why do some people think squalene in vaccines carries a risk?
- A few people have tried to link the health problems of Gulf War
veterans to the possible presence of squalene in the vaccines these
soldiers received.
- One published report suggested that some veterans who
received anthrax vaccines developed anti-squalene antibodies and these
antibodies caused disabilities.
- It is now known that squalene was not added to the vaccines
administered to these veterans, and technical deficiencies in the
report suggesting an association have been published.
More information
What is the relevance of anti-squalene antibodies and are these linked to squalene in vaccines?
- Most adults, whether or not they have received vaccines containing squalene, have antibodies against squalene.
- In one study the incidence of these antibodies appeared to increase with age.
- In one clinical trial, immunization with the licensed flu
vaccine containing squalene did not affect the frequency or titer of
anti-squalene antibodies.
(unpublished data shared with the GACVS by Novartis).
Reference for first bullet point above
Matyas
G, Rao M, Pittman P, Burge R, Robbins I, Wassef N et al. Detection of
antiboides to squalene III. Naturally occurring antibodies to squalene
in humans and mice. JIM 286 (2004) 47-67 Are squalene-containing vaccines safe?
- Over 22 million doses of squalene-containing flu vaccine have been
administered. The absence of significant vaccine-related adverse events
following this number of doses suggests that squalene in vaccines has
no significant risk. This vaccine has been given primarily to older age
groups.
- As this vaccine and new squalene-containing vaccines are
introduced in other age groups, post-marketing follow-up to detect any
vaccine-related adverse events will need to be performed.
|